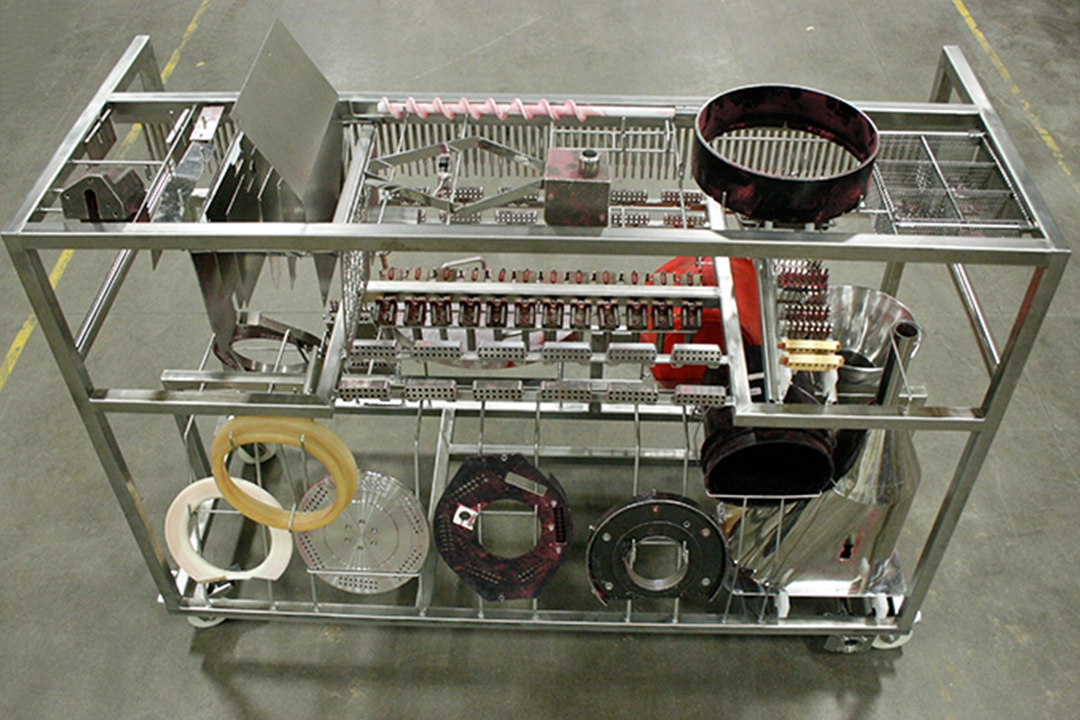
Designing a washer rack to clean pharmaceutical components—and make the cleaning validation process easier—requires a blend of science and art.
Because a validatable cleaning process is so important, customers frequently come to Sani-Matic for the custom racks in our ADEPT Rack Program that produce optimal, repeatable cleaning results.
“Our customers know what they need to do for a successful cleaning validation process, and they rely on us to provide them with washers and custom rack designs that will result in a validatable clean,” said Nikki Schoenbeck-Claas, Sani-Matic project engineer.

Nikki explained that racks, and washers, vary by application. “Some racks are meant to be used for cleaning glassware – spinners and beakers. Others are used for valves, clamps and gaskets. In many cases, the lightweight nature of the pharmaceutical items to be cleaned drive us toward a particular washer and rack design. For example, the lower spray pressures generated in our floor-mounted PharmaCab SP Series GMP cabinet washer are better suited for cleaning glassware than our larger cabinet washers.”
The importance of the process components to be cleaned in a particular load is why rack design begins with a customer-provided components list. Sani-Matic’s team works with the customer to determine item groupings and quantities. This establishes whether the list can be accommodated on one rack.
“In the beginning of the design process, our customers often do not know the dimensions of the components to be cleaned. If that is the case, we can pull from our experience with previous process components to begin the rack design, but the process is most successful if the components are shipped to us for 3-D modeling,” Project Manager Lavonne Dettmers said.
Chris McNulty, director of business development, Bio-Pharm added, “When a customer is unable to ship the components for 3-D modeling, we can send a field engineer to the customer site to measure and model their components.”
“Once we have the component information, we begin designing the racks and the blend of art and science comes into play,” noted Lavonne.
“Components are modeled and arranged in the available rack space using 3-D CAD software to position components for maximum spray coverage from the cabinet washer’s spray arms. We then add active piping with additional sprays to direct solution to the hard-to-clean surfaces that cannot be reached by the wash chamber’s spraying system,” added Lavonne.
“We plan the spray coverage using either cone- or fan-shaped sprays based on the pressure and flow provided by the washer pump. In some cases, we determine spray balls with targeted hole patterns deliver solution to specific locations more effectively. Careful planning when designing component layout and spray coverage increases the likelihood of a complete clean—and an easier cleaning validation process. But nothing can be guaranteed without testing,” she said.
The testing is what gives Sani-Matic, and its customers, cleaning confidence.
Sani-Matic Documentation and Tests Support Cleaning Validation
There are several tests and documentation Turn Over Packages (TOP) that help pharmaceutical companies achieve cleaning validation for both the washer and the racks. Here are three examples of tests Sani-Matic regularly performs:
- Riboflavin Coverage Test. Riboflavin, a yellow vitamin that glows under a blacklight, is sprayed throughout the washer’s chamber, tank, rack or other target items being tested. The rinse solution is then delivered through the washer’s sprays. Once a coverage test cycle is complete, the target item is checked with a blacklight to ensure the riboflavin has been completely removed.
- Factory Acceptance Test (FAT). An FAT is performed at Sani-Matic. Factory testing ensures the washer functions and meets the pharmaceutical-quality requirements as specified. Examples of functional requirements include the ability to use programmed recipes for cleaning cycles; being able to heat up cleaning solution within a specific time; and the use of instrumentation to enable verification of system pressure or flow. Quality requirements include material certifications.
- Site Acceptance Test (SAT). An SAT repeats the FAT testing process with the equipment installed at the customer’s facility. The SAT ensure the equipment operates as planned with onsite conditions. It is an important checkpoint because environmental and utility conditions, such as water supply temperature, ambient room temperature and steam capacity may vary and affect the system’s performance.
Pharmaceutical customers must perform extensive PQ (performance qualification) testing at their facility to guarantee the wash cycle has removed all contaminants and microorganisms to meet cleaning validation requirements. Occasionally, customers find that even though a rack was successfully covered by sprays, the contact was not rigorous or long enough to remove all soil down to the micro-level. In this case, they revisit the amount of cleaning time, chemical and temperature used and adjust as necessary.
Chris added, “If it is determined in the field that a certain component cannot be cleaned effectively due to nozzle positioning or other challenges, Sani-Matic offers a service to perform adjustments and rack modifications onsite to ensure a validatable clean.”
“The ingestible and injectable nature of pharmaceutical products mean our customers must adhere to the industry’s rigorous cleaning validation requirements,” stated Lavonne.
“Our tests are a great starting point for our customers,” Nikki said. “We work hard to make it easier for them to achieve cleaning success”