Bio-Pharm:
Clean-In-Place
(CIP) Systems
Critical Cleaning Solution, Validatable Results

Bio-Pharm: Clean-In-Place (CIP) Systems
Critical Cleaning Solution, Validatable Results
Clean-In-Place (CIP) Systems for the pharmaceutical, biotechnology, nutraceutical and personal care industries are automated systems used to clean the interior surfaces of manufacturing process pipes, vessels, tanks, bioreactors, fermenters, equipment and associated fittings, without disassembling the process. Thorough, repeatable in-place cleaning is critical to the quality of your product, the safety of consumers—and your bottom line.
Sani-Matic Clean-In-Place (CIP) Systems are engineered to your specific application, facility layout and utility requirements for effective and efficient sanitary process equipment cleaning. Our application-specific CIP design and sizing ensure sufficient flow and appropriate pressure is available to thoroughly remove residue, rinse effectively, shorten cycle times and promote worker safety.
Our in-house programming experts design each CIP program to optimize cycle times that get you back into production faster, ensure documentable results, while reducing chemicals, water use and operating costs.
Sani-Matic CIP Systems—from single- to multi-tank—are engineered to meet cGMP and ASME BPE standards.
Literature
View & download our latest manuals, technical datasheets, catalogs & more.
Articles
Read articles written by industry leaders to share their expertise and recommendations.
Solutions We Provide
UltraFlow®

Compact, portable, and cost effective.
Clean process tanks up to 10 ft in diameter.
Clean process lines up to 3″ in diameter.
TACTaFlow™
Standard, Pre-Engineered CIP Systems.
Clean process tanks up to 6 ft in diameter.
Clean process lines up to 2″ in diameter.
CIP Systems

Custom engineered to your specifications.
Fully automated and customized controls.
Meets cGMP and ASME BPE standards.
Sanitary Dryer L-HP

Standard Dry-In-Place (DIP) skidded system.
Dry sanitary process lines & small equipment.
Integrate with automated CIP or use standalone.
The Sani-Matic
Factory Acceptance
Test
Have you ever experienced a FAT where the manufacturer was not prepared and everything went wrong and it left you feeling disappointed and frustrated?
We promise that feeling will NOT be your experience with Sani-Matic – from the technical preparation to the hospitality, you’ll leave never wanting to FAT anywhere else!


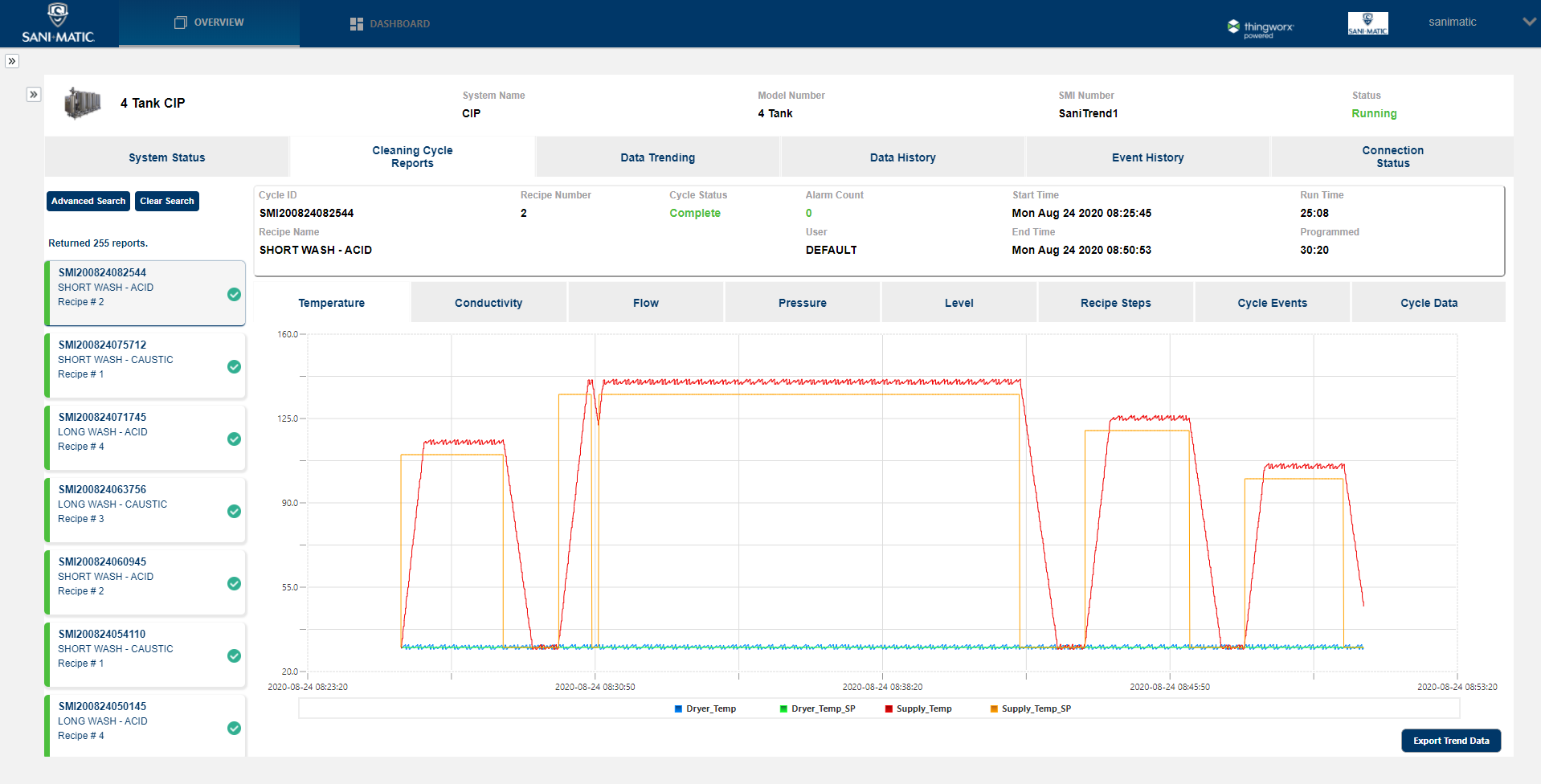
Automated Electronic CIP/COP
Data Recording, OEE, & More