High Purity
ALB Strainer
Industry Leading Process
Equipment Protection

High Purity ALB Strainer
Industry Leading Process Equipment Protection
Component and equipment protection is critical to keeping biopharmaceutical processes running – the High Purity ALB Strainer protects these components during CIP and other process steps, allowing production to continue as-designed, safely, and uninterrupted.
Designed to meet ASME BPE standards, the High Purity ALB Strainer is ready to integrate into your process today.
Resources
View brochures, manuals, technical datasheets, or watch videos for this product.
Articles
Read articles written by industry leaders to share their expertise and recommendations.
Product Resources
Literature
Videos
Product Details
Standard Features
○ 316Lss construction
○ Process contact finish: 20 µin Ra
○ Electropolished & passivated
○ Capture soils inside an easy to remove basket element
○ Patent pending end cap design reduces internal scratching & provides easy flushing of the basket element
○ Basket element can be removed without breaking process line
○ Designed for low pressure drops
○ Eccentric fittings for full drainability
○ Multiple inlet orientations & sizes
Optimized Design Utilizing Conceptual Fluid Dynamic (CFD) Modeling
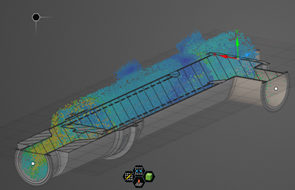
Capture Soils & Particulates Inside a Removable Element

Required Documentation (CDP-Premium Package)
○ Material Test Reports (MTRs)
○ Sani-Matic Certificate of Conformance (CoC)
○ Certified “As-Built” Drawings
○ Heat Map
○ Weld Map
○ Weld Log
○ Surface Finish Certification
○ Electropolish Certification
○ Passivation Certification