Bio-Pharm:
Custom CIP
Systems
Engineered to Your Specific Application.
Programmed to Integrate with Your Process.
Designed to cGMP and ASME BPE Standards..

Bio-Pharm: Custom CIP Systems
Engineered to Your Specific Plant Application and Utility Requirements
Sani-Matic Clean-In-Place (CIP) Systems are engineered to your specific plant application and utility requirements for effective and efficient process equipment cleaning. Sani-Matic’s CIP design and sizing ensure sufficient flow and appropriate pressure to thoroughly remove residue, rinse effectively, shorten cycle times, reduce operating costs, and promote worker safety. The CIP Systems meet cGMP and ASME BPE standards.
Single-Tank CIP Systems for Single-Use
There are many bio-pharm manufacturing processes that require a single-tank, single-use CIP system such as those that need to minimize the risk of cross-contamination, optimize the solution concentration for each cycle, meet industry critical cleaning requirements, or maintain portability through a smaller footprint.
This lower initial capital investment option provides simple and flexible once-through or re-circulated wash cycles, which are programmed by our in-house programming experts to meet your specific cleaning needs.
Whether cross-contamination, space constraints, industry requirements, or initial cost considerations are the reason for implementing a Sani-Matic single-tank, single-use CIP solution, it will provide you with the confidence your process is clean, every time.
Multi-Tank CIP Systems for Decreased Cycle Times
When time for cleaning is limited but space constraints and system portability are not a concern, bio-pharm manufacturing facilities may choose a multi-tank CIP system to decrease cycle times. Adding a second tank allows both the rinse and solution wash to be at the ready—no waiting for a single tank to refill, heat and charge with chemical following the initial rinse.
Sani-Matic Clean-In-Place (CIP) multi-tank systems are engineered to your specific plant application, layout and utility requirements and our in-house programming experts design each CIP program to optimize cycle times that get you back into production faster.
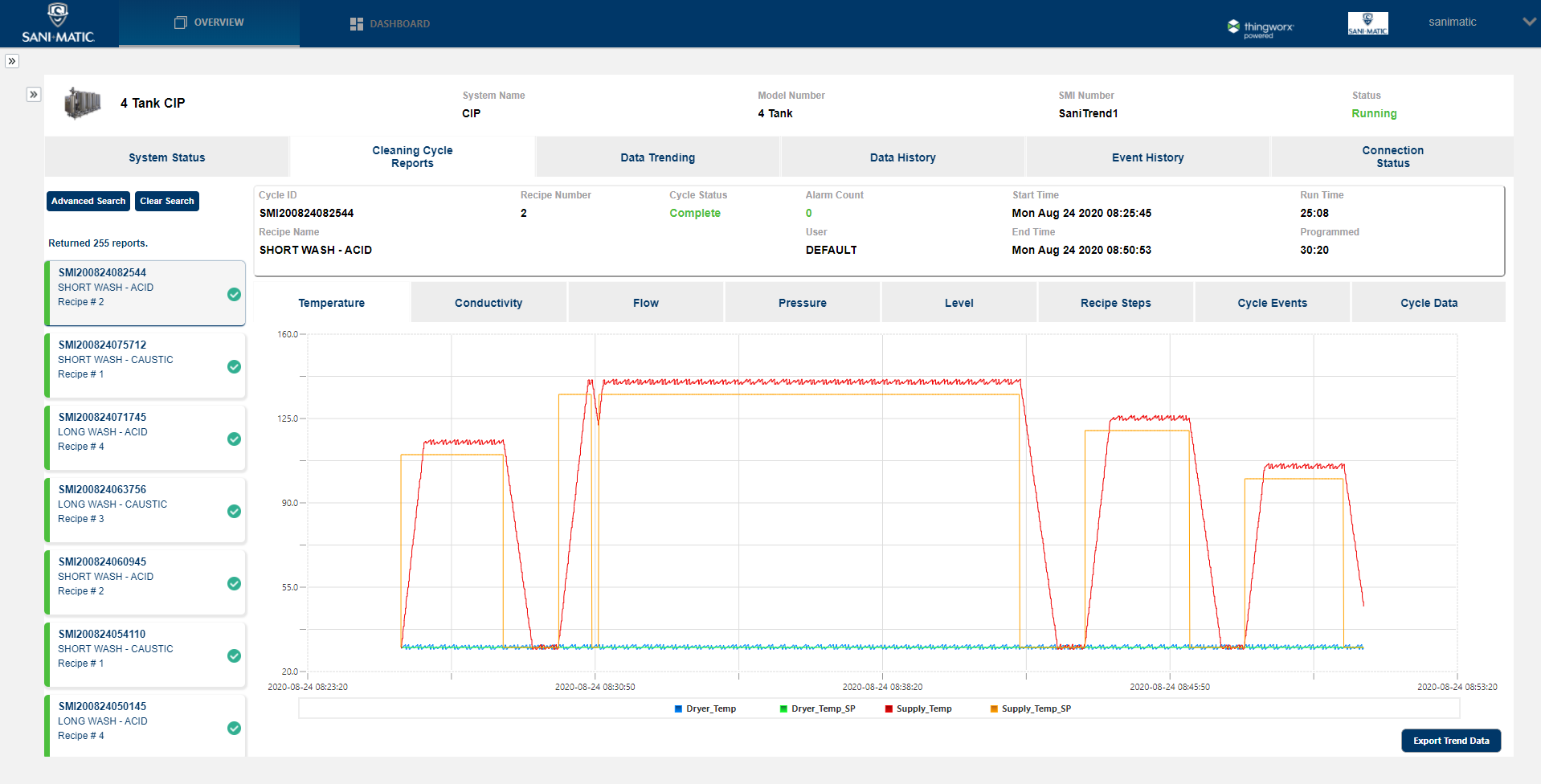
Available with SaniTrend ® Cloud CR Insights+ for Data Acquisition
SaniTrend Cloud provides automated, secure data acquisition and reporting of critical cleaning cycle information. The SaniTrend Cloud CR Insights+ tier is available as an option that is fully 21 CFR Part 11 Compliant, helping you meet reporting requirements for recording on cleaning processes. SaniTrend Cloud’s online functionality also offers additional system insights including Overall Equipment Effectiveness (OEE) trending, live dashboards, preventive maintenance trackers, event notifications, and more.
Resources
View brochures, manuals, technical datasheets, or watch videos for this product.
Articles
Read articles written by industry leaders to share their expertise and recommendations.
Product Resources
Literature
Videos
Product Details
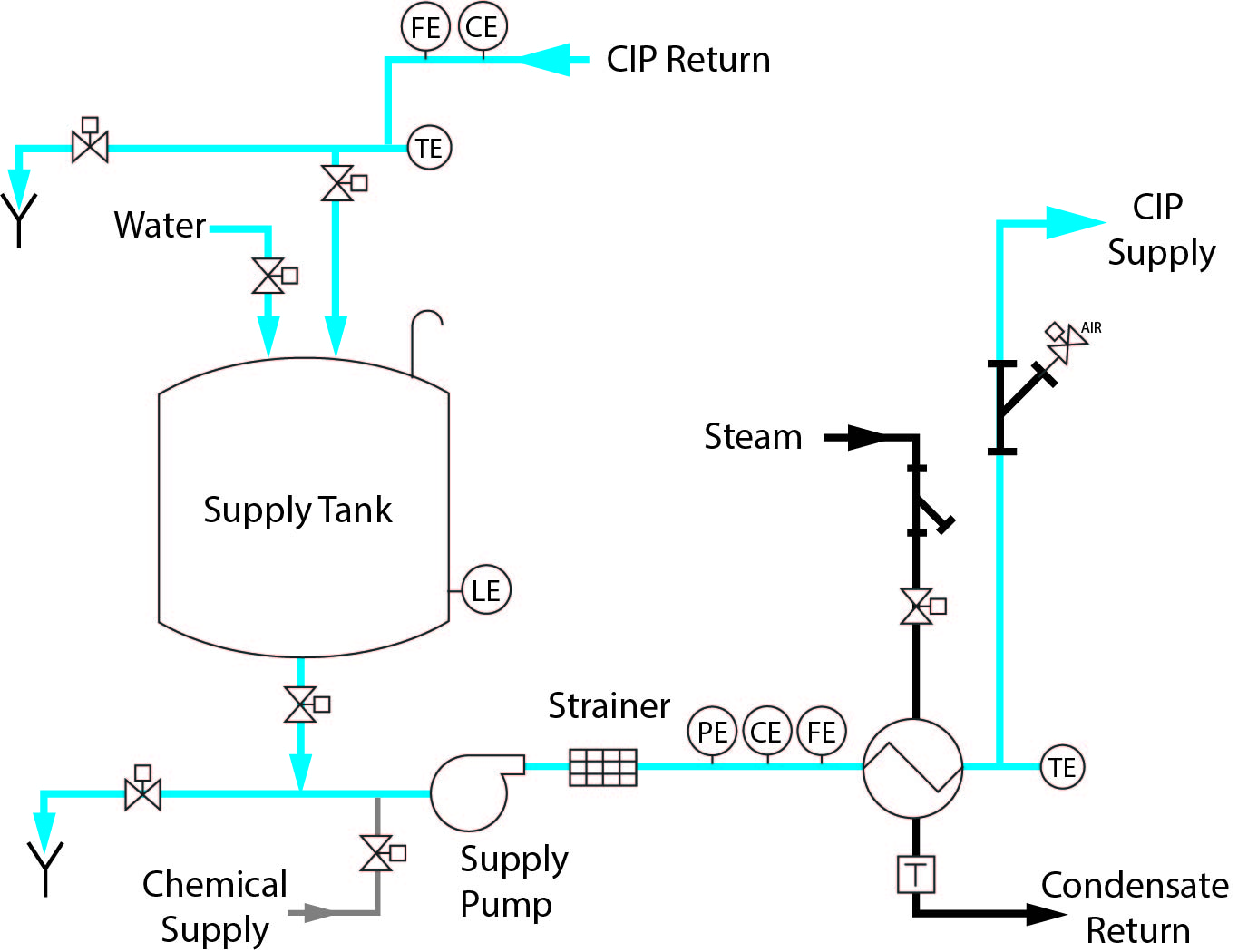
1 Tank CIP – Standard Features
○ Single-use source of cleaning solution and rinse water
○ Low capital investment
○ Portable or stationary design
○ Once-through or recirculated
○ Minimal space requirements
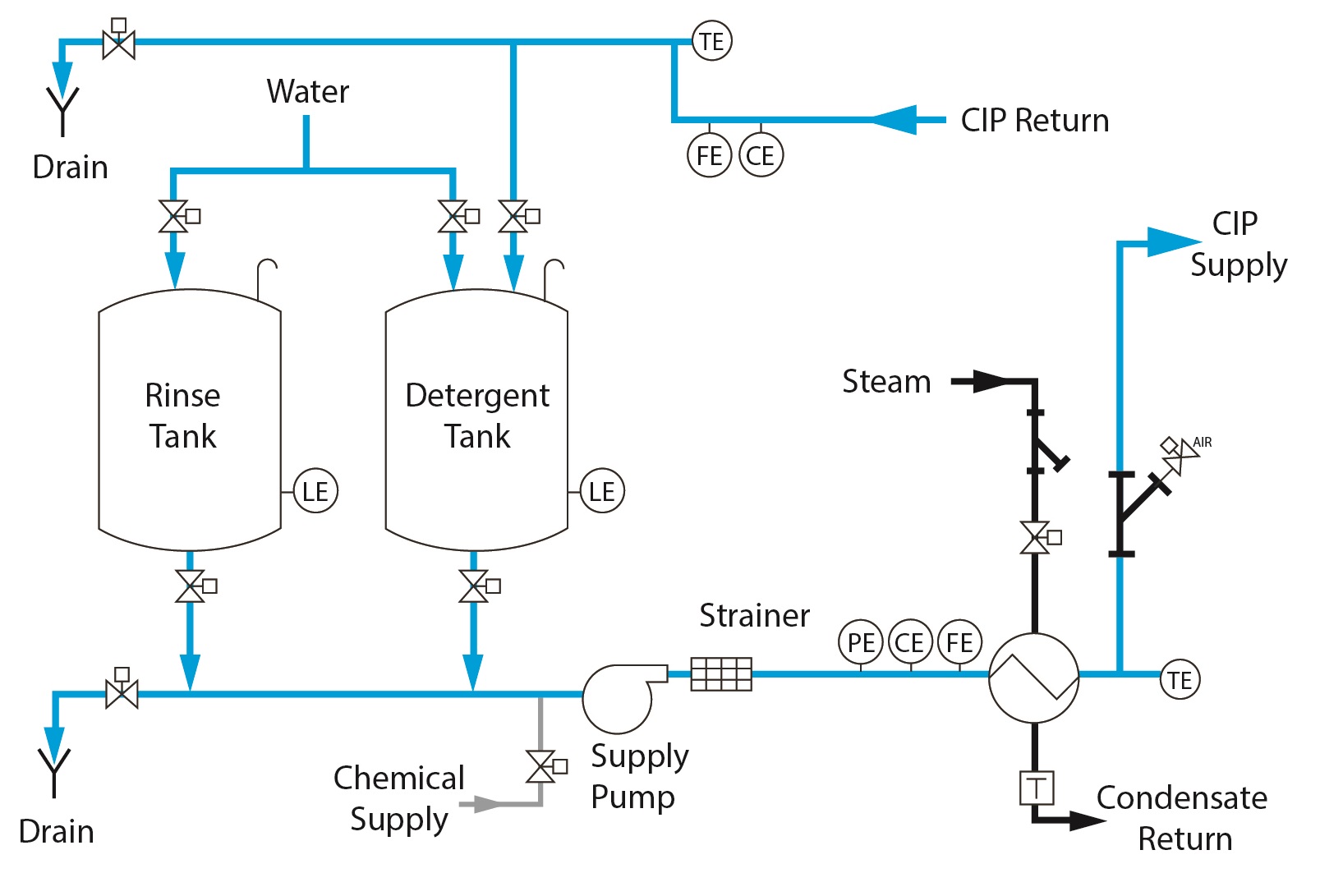
2 Tank CIP – Standard Features
○ Stationary Design
○ Dedicated rinse & wash tanks
○ Once-through or recirculated
○ Decreased cycle time over 1 Tank design

Automated Electronic CIP/COP
Data Recording, OEE, & More