Categories: Bio-Pharm, Food & Beverage, Personal Care & Nutraceuticals
Guidance to meet pharmaceutical manufacturing regulatory standards is everchanging, as is highlighted in the recent 2019 […]
Professionals in the bio-pharm industry understand meeting pharmaceutical equipment components requirements is a rigorous task. Close […]
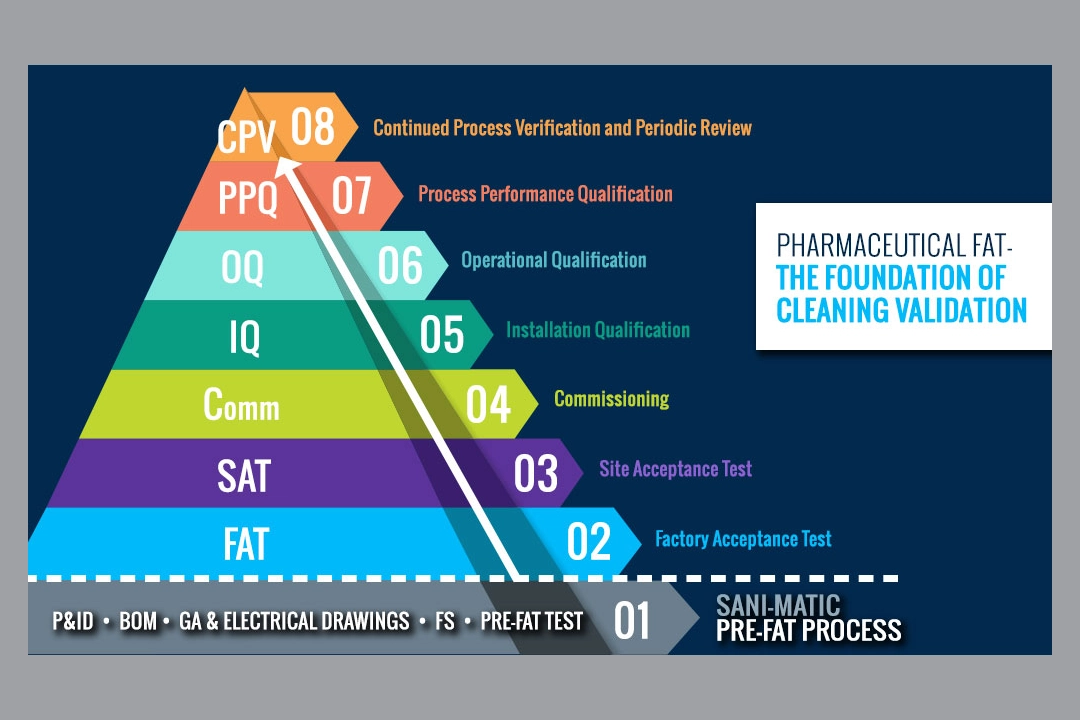
The Pharmaceutical Factory Acceptance Test (FAT) is the foundation of your validation process. Conceptually, one can […]
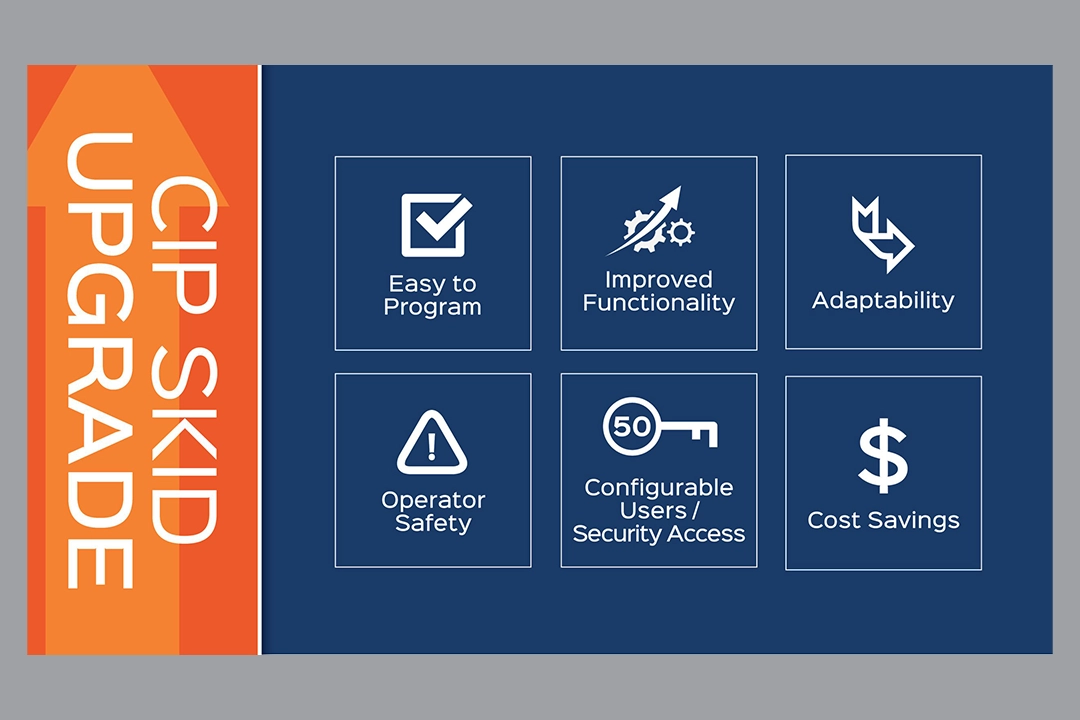
As food, beverage and pharmaceutical manufacturing processes change and operations grow, a CIP skid may require […]
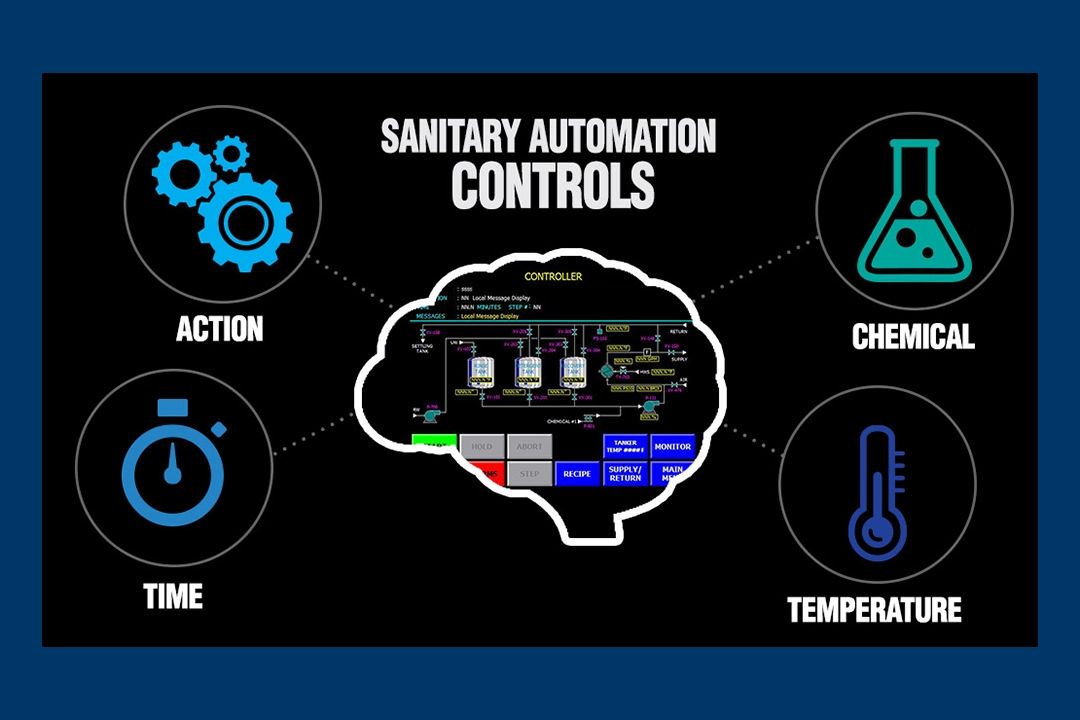
Sanitary automation controls are at the heart, and brain, of repeatable and customizable cleaning solutions. Without […]
Sanitary Components Catalog
[wp_blog_designer]