For years Sani-Matic has helped provide sanitary process solutions through automated cleaning equipment design and consultative service for pharmaceutical and other industries with stringent hygienic requirements.
But how are companies in these industries confirming the sanitary process solutions for cleaning are working? According to Justin Jaeck, vice president of technical sales, riboflavin testing is the benchmark for validating a complete spray coverage. “Riboflavin testing is most often used by our bio-pharm customers, but we are seeing a growing number of food processors using it as an evaluation tool to document the cleaning process is working.”
Riboflavin testing also provides clues of what may be causing contamination. Sani-Matic’s Field Service Technician Charles Bruce said, “Riboflavin is the only way to efficiently prove complete spray coverage, which is needed for a sanitary clean. You can perform swab testing, but depending on the product and swab test used, it may take up to one week to get results—and those rarely identify what the specific problem is. Riboflavin testing allows us to quickly identify the problem and provide a solution.”
Common Issues Affecting Cleaning
The most commonly diagnosed cleanability issues found by the Sani-Matic Tactical Solutions field engineers during riboflavin testing include the following:
- Clogged spray devices
- Spray patterns that don’t meet complex tank designs
- Incorrectly positioned or rotated spray devices
- Damaged spray device
- Spray patterns that are not designed to work with customer tank modifications
- Design, pressure and flow rate are inadequate for what’s identified
on the spray device(s) - Modified systems needing a new validation
- Baffles, agitators or other obstructions that cause shadowing or tough-to-clean areas
- Miscellaneous mechanical issues
Riboflavin Testing: The Part It Plays in Sanitary Process Solutions and Confirming Cleanability
A recent report from PMMI Business Intelligence shows food processors’ number one request of process equipment is cleanability [1]. Riboflavin testing plays its part in proving cleanability.
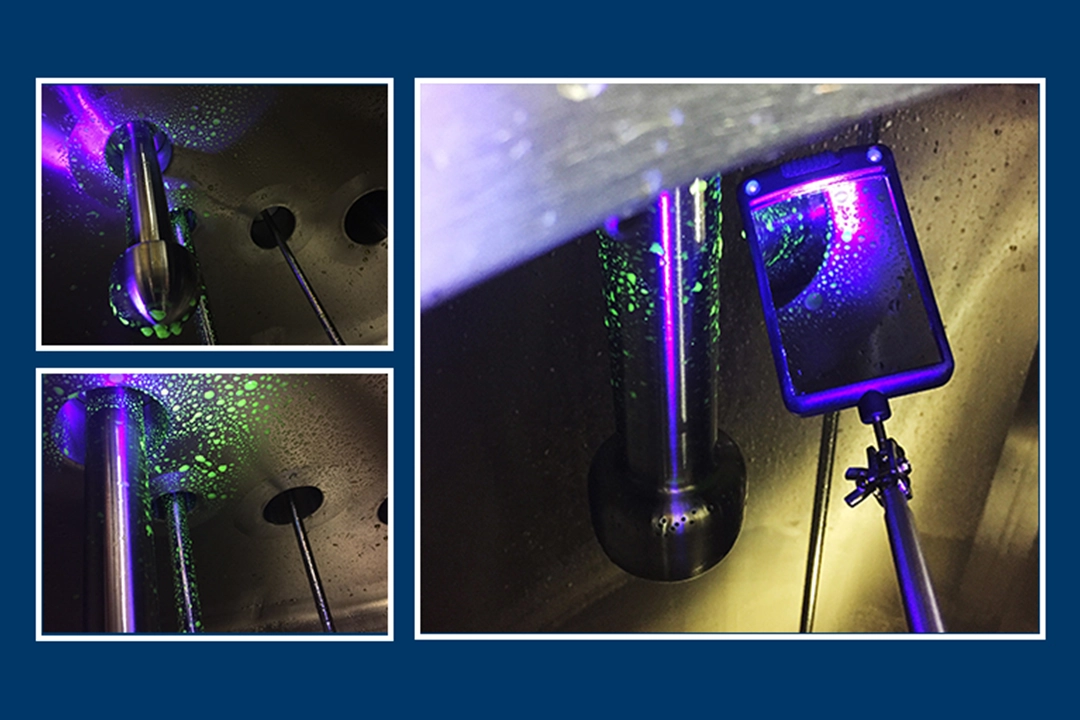
Testing involves coating all surfaces to be cleaned with a riboflavin solution. Riboflavin, a yellow vitamin that glows under a black light or UV-A light, is mixed with water and sprayed throughout the wash chamber, tank, rack or other target items being tested for cleanability.
A rinse solution is then delivered through the spray devices in a burst-rinse method. The burst-rinse method is a timed burst rinse followed by a timed gravity drain—repeated three times.
Once a coverage test rinse is complete, critical cleaning points are visually inspected using a black light and telescoping mirror to ensure the riboflavin solution has been completely removed. No visible fluorescence verifies complete spray coverage. If there is a residue showing under the black light, the field engineers can determine where the spray device coverage is not reaching.
Bruce emphasized, after the tank or equipment has been isolated and fully covered with the riboflavin solution, the cleaning cycle must be run within the limitations of the system’s design.
“When performing riboflavin testing, we need to be sure we are operating within the designed flow and pressure parameters to know the results are repeatable,” added Bruce.
Through visual inspection, the test confirms the spray pattern and device positioning are providing spray coverage to the targeted areas as designed. Once this baseline is established, the TACT variables—Time, Action, Chemical and Temperature—are introduced and tested to confirm a full, validatable clean.
If the Riboflavin Test Fails
Sani-Matic engineers will identify areas that may present a hygienic or cross-contamination risk and take corrective action before repeating the test. “One out of two jobs require some sort of modeling or information capturing, because we are often working from old drawings from before modifications or changes,” said Bruce. “Information capturing, such as internal geometries, agitator positions, port locations, etc., is critical because accurate measurements in the field are the foundation for the rest of what we do for sanitary process solutions.”
After modeling and information capturing, if it is determined a new spray device is required, 3-D models are created to identify the spray pattern needed to thoroughly clean the tank heads, walls and hard-to-clean obstructions.
“Once we’ve designed the proper spray device we can perform on-site riboflavin testing to ensure the spray pattern provides complete spray coverage. A passed riboflavin test serves as proof for our food and beverage customers that we have achieved that,” said Bruce. “However, any adjustments made to a bio-pharm customer’s cleaning system or process requires re-validation.”
Riboflavin Testing at Work
“We recently worked with a soft drink manufacturer whose lines passed cleaning inspection, however the system continued to fail microbial testing. Our field personnel performed riboflavin testing to diagnose the problem.”
The team inspected the tank and noticed that ports had recently been added. The original spray devices weren’t hitting correctly because flow and pressure measurements had been a matter of guesswork.
“Once corrective action was taken, we repeated riboflavin testing on the silos to document a sanitary clean,” Bruce said.
That is Cleaning Confidence.