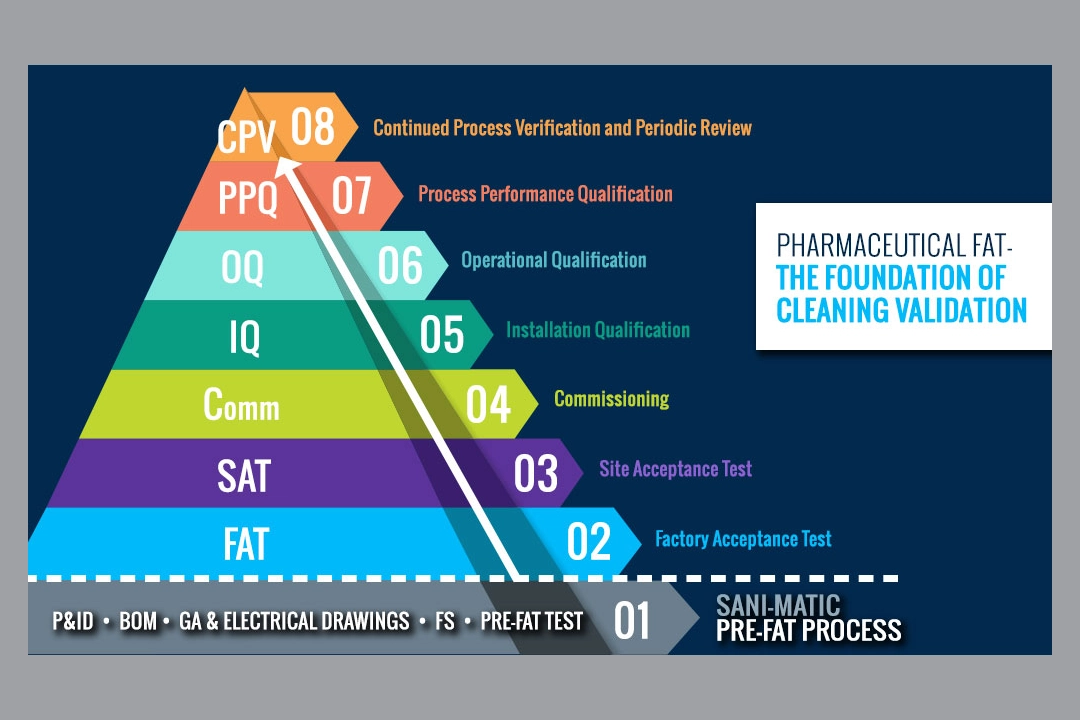
The Pharmaceutical Factory Acceptance Test (FAT) is the foundation of your validation process. Conceptually, one can see the validation process as a pyramid. And at the very base of the validation process pyramid is the FAT. From there the equipment is shipped to the customer site for the Site Acceptance Testing (SAT), then Commissioning, Installation/Operational Qualification (IQ/OQ), and finally the Process Performance Qualification (PPQ). For cleaning, this is where the ‘Cleaning Validation’ occurs. After that, the process moves into a monitoring phase for ongoing process verification and periodic review.
Because a strong foundation is needed to support large process structures, Sani-Matic has identified several crucial steps to complete before the pharmaceutical factory acceptance test stage to ensure the foundation is robust. Pete Barrie, product manager for the company, outlines the importance of getting the structural building blocks right early in the process.
Design and Review, Design and Review
“At the outset of a project, when we receive an RFQ, it is usually accompanied by the User Requirement Specification (URS), which is a bulleted, high-level description of what kind of equipment a customer is looking for and what it needs to do. Along with the URS there are detailed bid package documents to support it,” Barrie stated.
“After an order is received, we transition into our detailed design and review process. Our project engineers do an incredible amount of design work detailed in a number of documents,” he added.
Those documents include:
- P&ID – Piping and Instrumentation Diagram
- BOM – Bill of Materials
- Electrical Drawings
- General Assembly (GA) Drawings – 3-D models and 2-D drawings
“All of those drawings and documents are shared with the customer through a very detailed and collaborative review process,” Barrie continued. “During the review process, the customer can check against the initial URS and work with us to fine-tune everything, or further develop and define the system. After we receive customer approval of all the documents, we begin the fabrication process.”
Minimizing Costly Changes with Attention to Detail
It’s critical to work through the intricate design and functionality details at the outset of the project to avoid unnecessary costs and delays for the customer.
“The further along in the process you are, the more expensive, time consuming and difficult to make adjustments to a system,” Barrie emphasized. “For example, if you want to change from a one-tank to a two-tank CIP during the bid process, no problem, we’ll just requote to the new specifications. If we get to the design phase and the customer wants to make that change, we’ll need to take time to update the appropriate design documents, impacting both schedule and cost – and the extent of the impact is highly dependent on where we are in the design phase. If we are all the way to the FAT, it’s a big problem, considering the whole system has already been built.”
That concept is multiplied with each step up the validation process pyramid.
“If a customer gets to any onsite validation phase, and they realize a certain step was missed during the design review it becomes a nightmare for the customer. The system has already been built, gone through the pharmaceutical factory acceptance test, is already onsite and potentially has gone through several other steps. That’s why it’s so crucial to pay attention to every detail at the beginning of a project. Our project engineers are extremely detail oriented and work closely with our customers to avoid late-in-the-game changes as much as possible,” Barrie said.
The Structural Anchor for the Foundation
As a project moves into fabrication, Sani-Matic’s documentation specialists step in to develop the Functional Specification (FS) document. The FS is a precursor to the pharmaceutical factory acceptance test. It defines how the system is going to function based on all of the approved design documents provided by the project engineers. It includes all of the necessary documentation requirement details, recipes, sequence of operations and a comprehensive list of all the components in the system.
“The FAT is really the crescendo of our system development. There is a lot that we do leading up to it,” Barrie added.
The Sani-Matic Pharmaceutical Factory Acceptance Test Experience
An FAT is the culmination of an incredible amount of work and collaboration between all departments at Sani-Matic and the customer. Every person and detail is crucial to the process and it builds up to this critical check point before the equipment is shipped out to the customer’s facility.
“It’s really one of the most involved and highest priorities that we do here at Sani-Matic. The entire team is available to provide any support needed during the entire process,” said Curtis Williamson, senior electrical project engineer who has been with the company for over 10 years.
“It’s really one of the best parts of my job,” he shared. “I like to get away from the desk and interact with our customers. Our customers work hard. They put their phones away and become a part of our team. They also recognize that we have a great product and show their appreciation of that. It gives us all a genuine sense of accomplishment.”
Getting Down to Business
There is a lot to cover in the whole pharmaceutical factory acceptance test process. Customers are encouraged to have a couple representatives to tackle the various tasks needed to execute the full FAT.
“We have at least six binders full of documents and specifications to go through with the customer,” Williamson explained. “It works best if there are at least two individuals here, and ideally, one of them will also be a part of the onsite equipment setup and training. With two people, one individual can dive into the documentation requirements with our project engineer and verify that everything they need is included, while I work with the other representative to review the skid or piece of equipment and run through every functional detail.”
This hands-on part of the testing walks the customer through every aspect of the equipment to ensure the customer understands how it works and what the equipment can do for them. A sample of typical items tested and checked in the process are:
- Thorough HMI (operator interface) review – Verify that all of the buttons and sequencing are correct. Review all of the screens to understand how to navigate and run the equipment.
- Functional Testing – Check each component and instrument to confirm everything is running to their setpoints and that all of the limits are correct.
- Performance Testing – Run through the mechanical verifications and cross-reference everything with the P&ID, BOM, GA and Electrical Drawings.
- Review Operation Codes and Sequencing – Run through every recipe, operation code (OpCode), opcode editing and verify that everything is sequencing properly in the PLC.
- Review Alarms and Messages – Execute all alarms and review all information messages on the HMI.
- Mechanical Verification – Check surface finishes and piping slopes, BOM and P&ID tagging verification, dimensional check.
“We review everything with a fine-tooth comb,” said Barrie. “We hold a pre-FAT two weeks prior to the FAT to ensure the system is working exactly as it is outlined within the Functional Specifications. That way the necessary adjustments are made before the customer even arrives at Sani-Matic for the actual FAT.”
Williamson added, “We hear all of the time from our customers that our FATs are the most thorough they’ve ever encountered. We want to make sure that this sets the stage for a smooth and efficient validation process for them. If any issues arise during the execution of the pharmaceutical FAT, we create a Punch List of those items and they are either addressed while the customer is here, or we’ll make sure to take care of them before the equipment is shipped.”
Sani-Matic Hospitality
Williamson says feedback regarding the Sani-Matic pharmaceutical factory acceptance test process is overwhelmingly positive and he attributes a lot of it to the hospitality set forth companywide.
“Customers really enjoy our hospitality and overall support we provide during the process. Customers have shared their experiences elsewhere and said they’ve been dropped off at the site of the equipment and they fend for themselves. Here, there’s always someone available and at their side. If they have any questions or concerns, we can address them in the moment and provide additional training,” he said.
“We truly go above and beyond for our customers. Even outside of business hours, our team looks for ways to get together and make sure they know where to go while they’re here. We’ll take them out to dinner and give them the chance to experience the charm of Madison. A thorough pharmaceutical FAT is an intense work week; we want to make sure they get a chance to enjoy themselves, too.”